Abstract
Background/Objective: The association between paroxysmal nocturnal hemoglobinuria (PNH) granulocyte clone size at disease onset and outcomes in patients with PNH remains unclear. Most but not all reports examining the relationship have shown a positive correlation between clone size and thrombotic events [TEs] in patients with PNH, but without a clear temporal association showing the prognostic value of clone size on the risk of TEs. The ongoing International PNH Registry (NCT01374360) is the largest prospective, observational study of patients with PNH conducted to date. The objective of this analysis was to examine the relationship between PNH granulocyte clone size at disease onset and risk of thrombosis after disease onset while untreated with a complement inhibitor in patients enrolled in the Registry.
Methods: The current analysis included patients enrolled in the Registry as of April 2018 who had known demographics, were untreated with complement inhibitor therapy at enrollment, and had ≥12 months of untreated follow-up after disease onset. Baseline was defined as disease onset (earliest of a reported PNH clone, date of PNH diagnosis, or reported PNH symptom), and patients were stratified into 5 cohorts based on clone size at baseline (using the earliest reported clone prior to enrollment): cohort 1, clone size: 0.01-1%; cohort 2, clone size: >1-5%; cohort 3, clone size: >5-10%; cohort 4, clone size: >10-50%; cohort 5, clone size: >50%. Event rates for TEs and all major adverse vascular events (MAVEs; including TEs) were calculated for the time period from baseline to last follow-up. Other outcomes of interest included LDH ratio (LDH/LDH upper limit of normal [ULN]), hemoglobin levels, platelet counts, absolute neutrophil counts, and absolute reticulocyte counts at last follow-up.
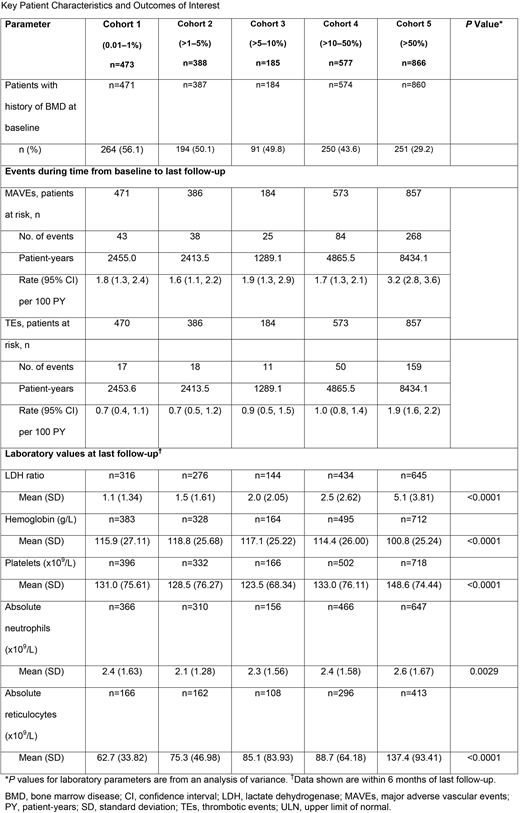
Results: A total of 2489 patients were eligible for the analysis. The majority of patients in the overall study population were female (54.0% [1343/2489]) and white (79.2% [1967/2484]). Mean (standard deviation [SD]) age at PNH start ranged from 36.4 (16.53) in cohort 5 to 44.2 (20.70) in cohort 1. Median time from baseline to last follow-up was 3.7 years in cohort 1, 4.3 years in cohort 2, 4.7 years in cohort 3, 5.6 years in cohort 4, and 6.8 years in cohort 5. Results for the outcomes of interest are summarized in the Table. All cohorts showed a risk of MAVE and TE during follow-up. Although estimated rates of MAVE and TE were highest in the >50% clone size cohort, there was no difference in the rate of MAVE or TE during follow-up across the 4 cohorts with clone size <50% at disease onset. Mean LDH ratio (LDH/LDH ULN) at last follow-up showed a statistically significant difference by clone size at baseline among the cohorts, ranging from a mean (SD) of 1.1 (1.34) in the patients with clone size <1% and increasing to 5.1 (3.81) in the patients with clone size >50% (P<0.0001). No difference in hemoglobin level at last follow-up was observed in the smaller clone size cohorts, although mean hemoglobin level was lower in patients with clone size >50% (P<0.0001). Similar trends were seen in mean platelet and absolute neutrophil counts across the smaller clone size cohorts, while patients with clone size >50% showed higher values (P<0.0001). Mean absolute reticulocyte count at last follow-up was lowest in patients with clone size 0.01-1%, and were incrementally higher in each successive clone-size cohort (P<0.0001).
Conclusions: In this study, all patients with a PNH clone at disease onset were at risk for TEs and other MAVEs. Patients in the highest baseline PNH clone size strata (clone size >50%) had an approximately 2-times higher risk of TEs than patients with smaller clone sizes; Patients with small clone sizes (0.01-1%) were older and showed a higher prevalence of BMD. There was no difference in the prognostic value of clone size at disease onset on the risk of TEs and other MAVEs in patients with small (0.01-1% and 1-5%) versus medium-sized (5-10% or 10-50%) clones.
Peffault De Latour:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding; Amgen Inc.: Research Funding. Maciejewski:Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Ra Pharmaceuticals, Inc: Consultancy; Ra Pharmaceuticals, Inc: Consultancy. Kulasekararaj:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support . Larratt:Alexion Pharmaceuticals, Inc.: Honoraria, Research Funding. Dingli:Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.. Wilson:Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Gustovic:Alexion Pharma GmbH: Employment, Equity Ownership. Kulagin:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal